What is Enterra® Therapy?
Discover a different kind of treatment
If you’re like many people living with gastroparesis, you’ve tried a number of treatment options—from diet modifications to medications and beyond—and still struggle to find relief. That’s why we developed Enterra® Therapy.
Answer a few questions to see if Enterra Therapy may be right for you.

The Enterra Therapy difference
The Enterra System is the first and only device specifically designed to relieve the nausea and vomiting associated with gastroparesis from diabetes or unknown origins by gently stimulating your stomach — a unique kind of therapy called gastric electrical stimulation (GES).
Unlike other surgical treatment options, Enterra Therapy is:
Implantable
The Enterra neurostimulator is placed just beneath the skin, usually in the lower abdominal region
Customizable
Your doctor will non-invasively adjust your system to help find the level of stimulation that’s right for you
Reversible
If Enterra Therapy isn’t right for you, your doctor can turn off or remove your system
How does Enterra Therapy work?
The Enterra System is made up of three parts:

A small neurostimulator that’s implanted under the skin
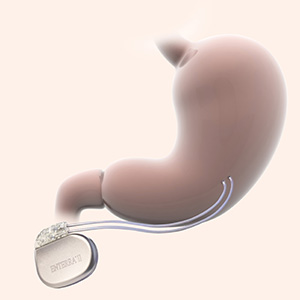
Two wires, called “leads,” that connect the neurostimulator to the stomach’s muscles

A handheld, external programming device
Once it’s implanted, the neurostimulator sends mild electrical pulses through the leads to gently stimulate the smooth muscles of the lower stomach. These pulses are designed to help control the chronic nausea and vomiting associated with gastroparesis*.
Using the programming device, your doctor will adjust the neurostimulator to help ensure you receive the level of stimulation that’s right for you. Adjusting your Enterra System’s level of stimulation is non-invasive and does not require surgery.
If you choose, you can ask your doctor to turn off your Enterra System, which does not require any additional procedures, or choose to have your system surgically removed.
Review more information regarding the probable benefits and risks associated with receiving an Enterra System.
Frequently asked questions
How large is the Enterra neurostimulator?
The Enterra® II Neurostimulator model 37800 is 2.4 inches (60 mm) long, 2.2 inches (55 mm) high, and 0.5 inches (11.4 mm) thick. It weighs 1.6 ounces (45 grams).
Will the Enterra neurostimulator eliminate my nausea and vomiting symptoms?
Enterra Therapy has 5 randomized control trials completed and over 20 years of clinical research. You should speak with your doctor regarding the clinical data for Enterra Therapy.
Among the patients that do experience relief, results vary. Often a combination of treatment options is necessary to maintain relief.
If gastric electrical stimulation works for me, when will I notice improvements in my symptoms?
The rate of improvement varies from person to person.
How long will my neurostimulator battery last?
How long the battery lasts depends on your stimulation settings. Some people need more stimulation, which drains the battery faster, and others need less.
What will happen when the neurostimulator battery runs down?
Your doctor will need to schedule a surgical procedure to replace the neurostimulator. The Enterra II Neurostimulator contains a battery indicator that tells your doctor when it is time to schedule a device replacement for continued therapy.
Can the neurostimulator battery be recharged?
The entire neurostimulator, which contains the battery, must be replaced when the battery runs down. The neurostimulator is not rechargeable.
Will I be able to turn the neurostimulator on and off?
Only your doctor can turn the neurostimulator on and off with the external clinician programmer.
Can the Enterra neurostimulator be used during pregnancy?
The safety of neurostimulation for use during pregnancy or delivery has not been established. If you learn, or suspect, that you are pregnant, call your doctor.
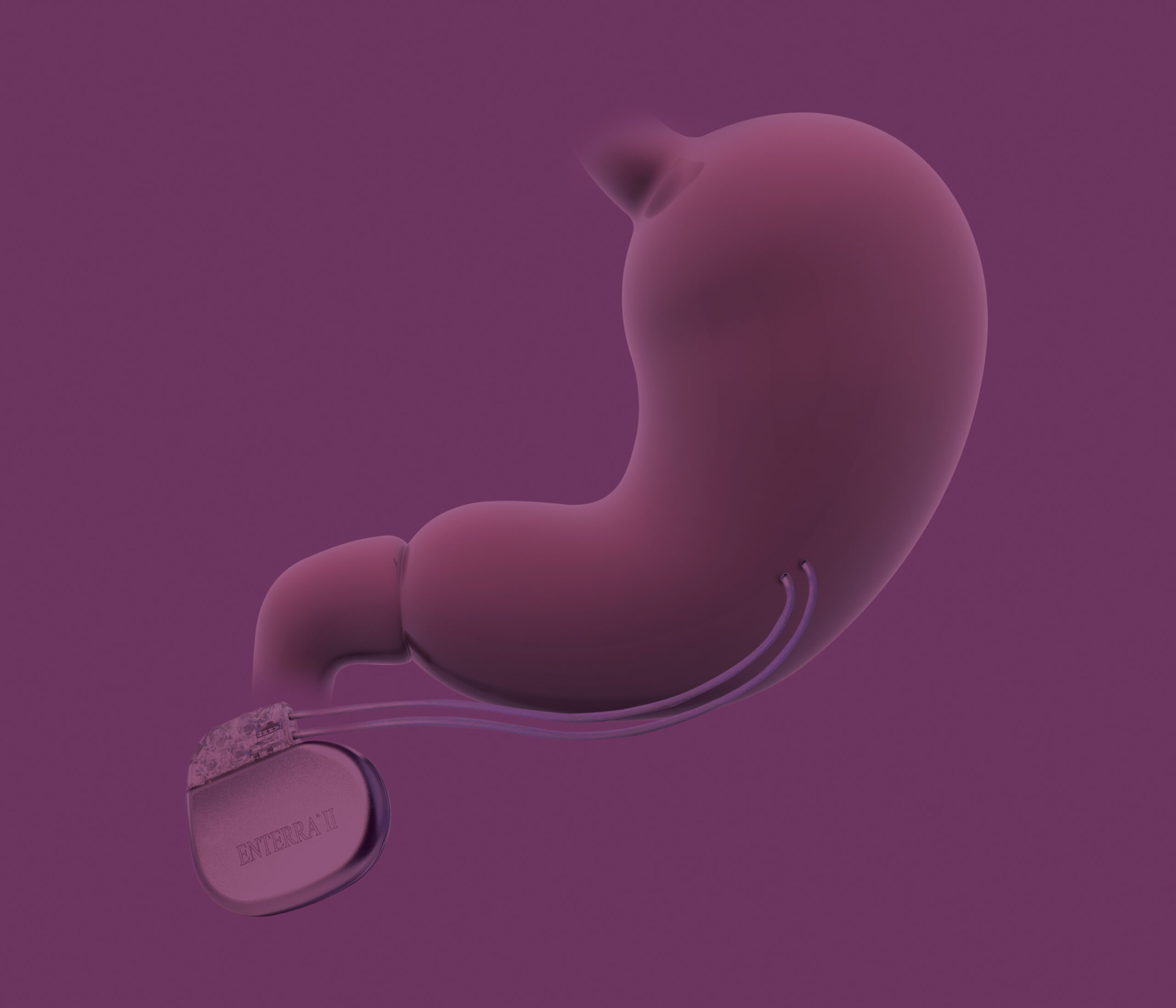
Enterra Therapy is a humanitarian device
A humanitarian device is a medical device specially designated by the US Food and Drug Administration (FDA) for use in the treatment of a rare medical condition (fewer than 8000 new cases per year in the United States). The FDA requires that any physician who wishes to use the device to treat a patient must first obtain approval from the hospital’s institutional review board. The Enterra Therapy System was designated as a device for humanitarian use by the FDA in 1999.
After Enterra Therapy was designated for humanitarian use, a humanitarian device exemption application was submitted to the FDA. This type of application is not required to contain the results of scientifically valid clinical investigations demonstrating that the device is effective for its intended purpose. However, it must contain sufficient information for the FDA to determine that the device does not pose an unreasonable or significant risk of illness or injury and that the probable benefit to health outweighs the risk of injury or illness from its use, taking into account the probable risks and benefits of currently available devices or alternative forms of treatment. Also, the applicant must demonstrate that no comparable devices are available to treat or diagnose the disease or condition, and that the applicant could not otherwise bring the device to market. Once the FDA approved the Humanitarian Device Exemption in 2000, the Enterra Therapy System was then manufactured and distributed in the United States.
The information provided on this site is for general educational purposes only and is not a substitute for professional medical advice, diagnosis or treatment. Always talk to your doctor about the best treatment options for your individual situation.
Disclaimer: This page may include information about products that may not be available in your region or country. Please consult the approved indications for use. Content on specific Enterra Medical products is not intended for users in markets that do not have authorization for use.
IMPORTANT SAFETY INFORMATION
Enterra Therapy for treatment of chronic, resistant to medication nausea and vomiting associated with gastroparesis caused by diabetes or an unknown origin in patients aged 18 to 70 years: patients should always discuss potential risks and benefits of the device with their physician.
*HUMANITARIAN DEVICE
Authorized by Federal law for use in the treatment of chronic intractable (drug refractory) nausea and vomiting secondary to gastroparesis of diabetic or idiopathic etiology in patients aged 18 to 70 years. The effectiveness of this device for this use has not been demonstrated. What does this mean?


