The Enterra® Therapy difference
A different approach to managing gastropararesis
Enterra® Therapy is the first and only device designed to reduce the nausea and vomiting associated with diabetic or idiopathic gastroparesis through gastric electrical stimulation (GES).
Implanted in over 15,000 patients, it is an advanced therapy option for gastroparesis patients in their journey to find relief.
Targets nausea
& vomiting
Adjustable
Reversible
Minimally invasive
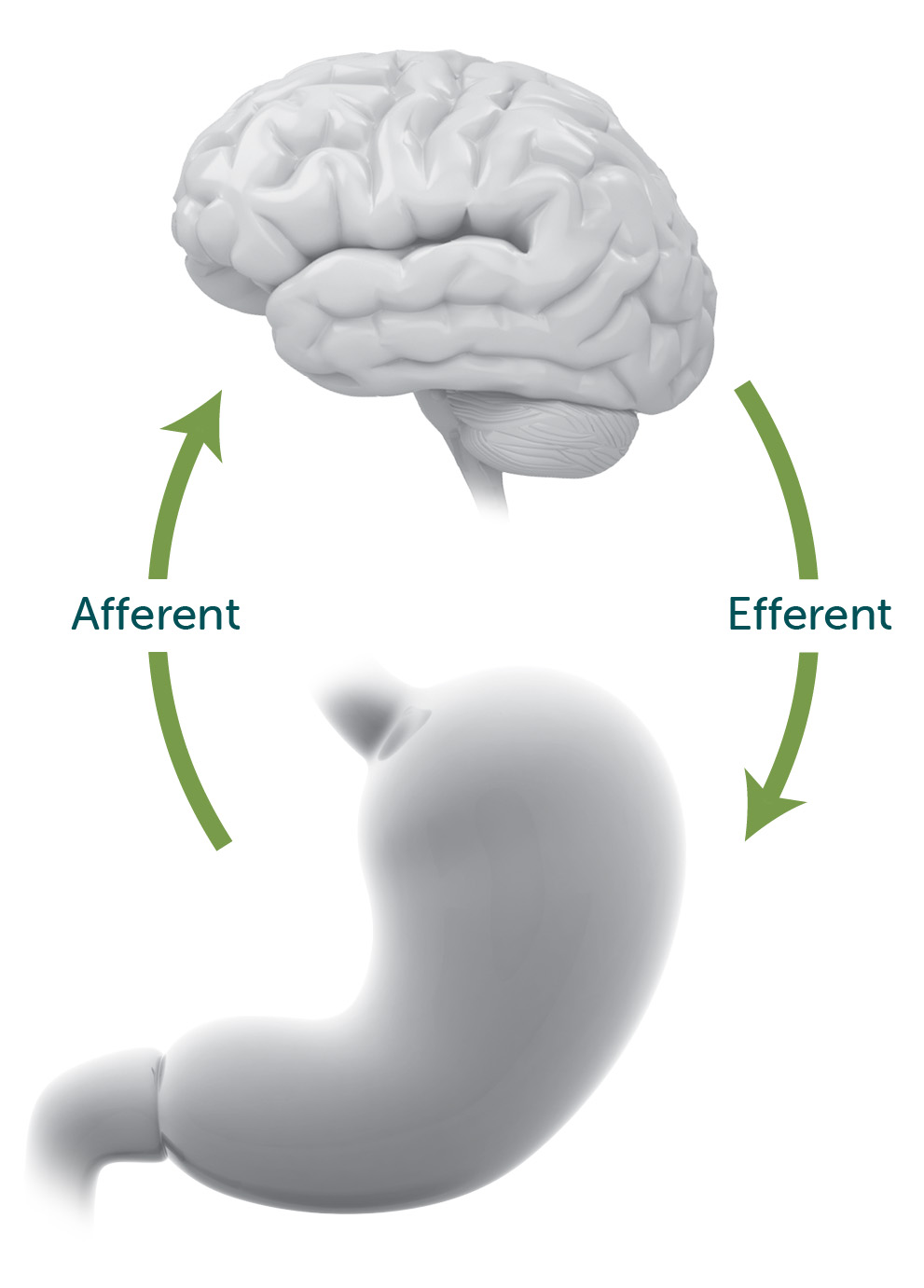
Gastric Electrical Stimulation: Potential Mechanism of Action
The exact mechanism of action of GES is unknown, but the AGA clinical practice update on the potential mechanism hypothesizes that Enterra Therapy may impact the afferent (sensory) and efferent (motor) pathways between the stomach and central nervous system, the cell types found in the circular muscle (ICC-CM), and myoneural connections–allowing for the alleviation of symptoms.1
How Enterra Therapy works
Enterra Therapy stimulates the nerves and smooth muscles of the stomach by delivering mild electrical pulses, thereby reducing nausea and vomiting symptoms associated with diabetic and idiopathic gastroparesis.
Neurostimulator

A small, battery-powered gastric neurostimulator is implanted beneath the skin in the lower abdominal region.
Leads
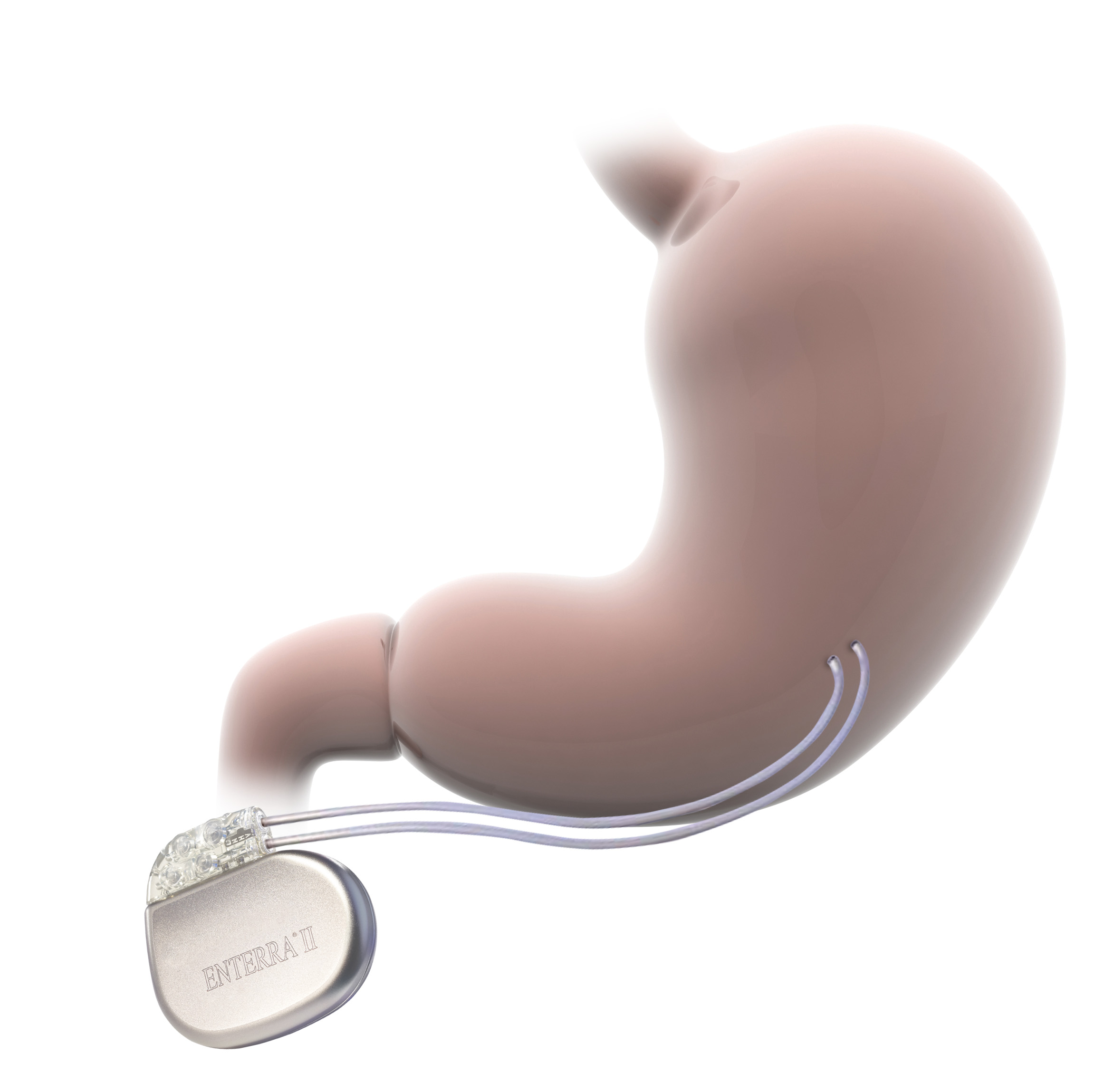
Leads deliver mild, controlled electrical pulses to the antrum portion of the stomach muscle wall.
Programmer

The system is programmed to optimize therapy for the individual patient.
Unlike other surgical options, therapy with GES is reversible and preserves the patient’s innate anatomy. Using the external clinician programmer, therapy can be turned on or off at any time without surgery. The device can also be removed from the patient’s body.
Procedure overview
Electrodes are implanted in the stomach wall
Enterra Therapy implant procedure typically takes 1-2 hours in a minimally-invasive laparoscopic or robotic surgical procedure, where the leads are placed on the serosal surface of the stomach’s greater curvature.
Neurostimulator is implanted in subcutaneous abdominal pocket
The neurostimulator is placed in subcutaneous tissue, typically in the abdomen.
Patient recovery is typically 1-2 days
While some patients leave same-day, a 1-2 day hospital stay is typical.
Therapy is adjusted non-invasively
Post-procedure, Enterra Therapy can be adjusted for symptom control via a programmer in an outpatient clinic.
Indication
Enterra Therapy is indicated for use in the treatment of chronic, intractable (drug refractory) nausea and vomiting secondary to gastroparesis of diabetic or idiopathic etiology in patients aged 18 to 70 years.
FDA-approved as humanitarian device
GES with Enterra Therapy has been FDA approved as a humanitarian device since 1999.
A humanitarian use device is a medical device intended to treat or diagnose a disease or condition that affects, or is manifested in, fewer than 8,000 individuals a year in the United States. Approval is based on a determination that the device is safe and has probable benefit. Humanitarian use devices must be implanted in a medical center whose institutional review board has approved use of the device.
Manuals and technical guides
Access prescriber, implant, MRI, and other manuals on the manual library.
- Lacy BE, Tack J Gyawali CP, AGA Clinical Practice Updated on Management of Medicall Refractory Gastroparesis: Expert Review [published online ahead of print, 2021 Oct29] Clin Gastroenterol Hepatol. 2021;S1542-3565(21)011151-4.
doi:10.1016/j.cgh.2021.10.038.
MKT-D-0006, Rev N
IMPORTANT SAFETY INFORMATION
Enterra Therapy for treatment of chronic, resistant to medication nausea and vomiting associated with gastroparesis caused by diabetes or an unknown origin in patients aged 18 to 70 years: patients should always discuss potential risks and benefits of the device with their physician.
HUMANITARIAN DEVICE
Authorized by Federal law for use in the treatment of chronic intractable (drug refractory) nausea and vomiting secondary to gastroparesis of diabetic or idiopathic etiology in patients aged 18 to 70 years. The effectiveness of this device for this use has not been demonstrated. What does this mean?
