Challenge of gastroparesis
Gastroparesis is more than a motility issue
Correlation between delayed gastric emptying and cardinal symptoms are not well established in gastroparesis.1
Current treatments focused on addressing motility often fail, leaving gastroparesis patients without options focused on relieving nausea and vomiting—the most distressing symptoms of gastroparesis.2
Enterra® Therapy is the first and only device designed to reduce the nausea and vomiting associated with diabetic or idiopathic gastroparesis through gastric electrical stimulation (GES).
In 2021, a 12-year NIH study of patients with upper GI symptoms found gastric emptying results are variable and do not correlate with clinical symptoms.3
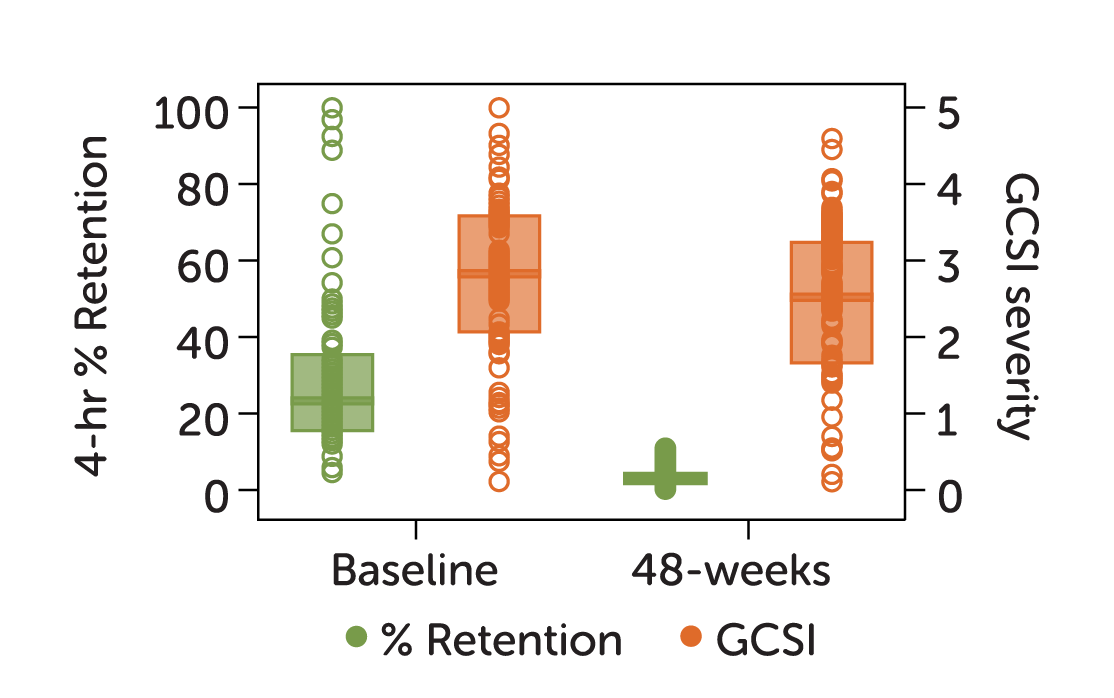
42%
of patients showed emptying improvement without significant improvement in symptoms
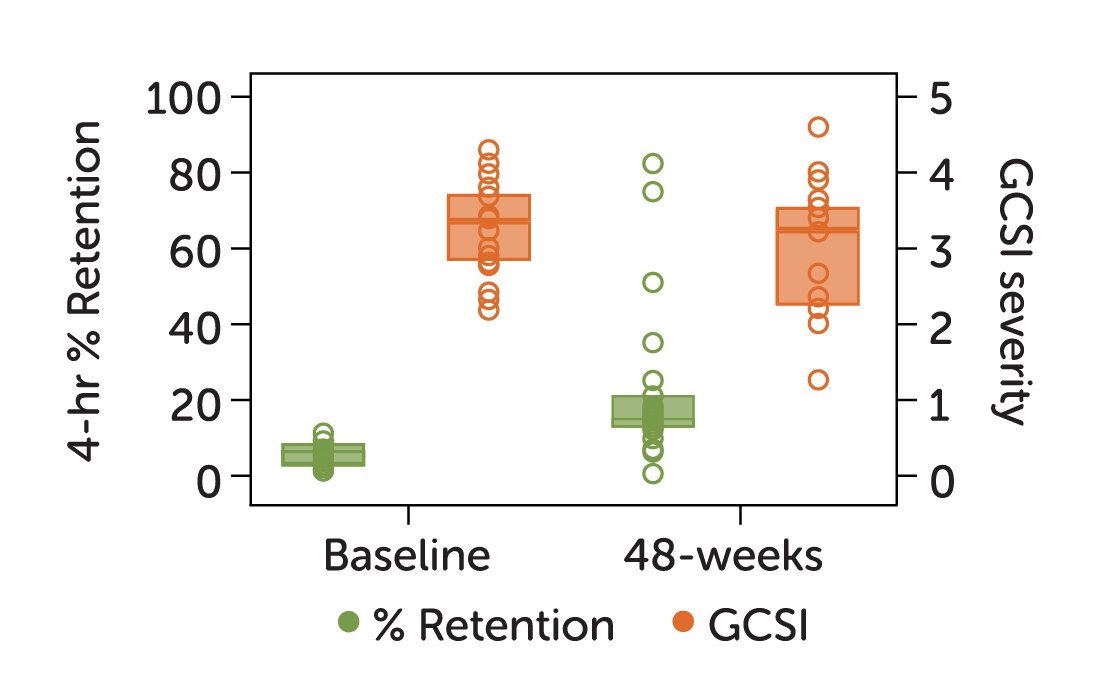
37%
of patients showed emptying worsening without significant change in symptoms
Gastroparesis is a quality of life issue
Although it takes an average of 5 years from the onset of symptoms until diagnosis, the journey of gastroparesis hardly ends there — for patients or providers.2
Between an evolving understanding of the disease’s characteristics and pathophysiology and limited treatment options, gastroparesis remains challenging to treat.
In a recent survey of 1,423 gastroparesis patients, only 4% reported that they were satisfied with available treatment options.2
The exhaustion, isolation, and frustration of gastroparesis is real and costly:
68%
Reduction in daily activities4
29%
Lower annual income and higher rates of unemployment and underemployment4
11%
Medically disabled4
24%
Combined anxiety/depression4
- Janssen P, Harris MS, Jones M, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic
and idiopathic gastroparesis. The American journal of gastroenterology. 2013;108(9):1382-1391. doi:10.1038/ajg.2013.118. - Yu D, Ramsey FV, Norton WF, et al. The Burdens, Concerns, and Quality of Life of Patients with Gastroparesis. Dig Dis Sci .
2017;62(4):879-893. doi:10.1007/s10620 017 4456-7. - Pasricha, J. et al. Functional Dyspepsia and Gastroparesis in Tertiary Care are Interchangeable Syndromes With Common Clinical and Pathologic Features. Gastroenterology. 2021 May; 160(6):2006-2017. doi: 10.1053/j.gastro.2021.01.230.
- Lacy BE, Crowell MD, Mathis C, Bauer D, Heinberg LJ. Gastroparesis: Quality of Life and Health Care Utilization. J Clin Gastroenterol.
2018;52(1):20-244.doi:10.1097/MCG.0000000000000728.
IMPORTANT SAFETY INFORMATION
Enterra Therapy for treatment of chronic, resistant to medication nausea and vomiting associated with gastroparesis caused by diabetes or an unknown origin in patients aged 18 to 70 years: patients should always discuss potential risks and benefits of the device with their physician.
HUMANITARIAN DEVICE
Authorized by Federal law for use in the treatment of chronic intractable (drug refractory) nausea and vomiting secondary to gastroparesis of diabetic or idiopathic etiology in patients aged 18 to 70 years. The effectiveness of this device for this use has not been demonstrated. What does this mean?
