US control trial: diabetic gastroparesis1
This trial examined the safety and efficacy of Enterra Therapy in the treatment of chronic, intractable (drug-refractory) nausea and vomiting secondary to gastroparesis of diabetic etiology.
A number of key clinical studies involving diabetic and idiopathic gastroparesis patients receiving Enterra® Therapy resulted in significant nausea and vomiting symptom improvements.

The largest, independently conducted, prospectively collected long-term follow-up data series on Enterra Therapy use in the US for patients with gastroparesis.
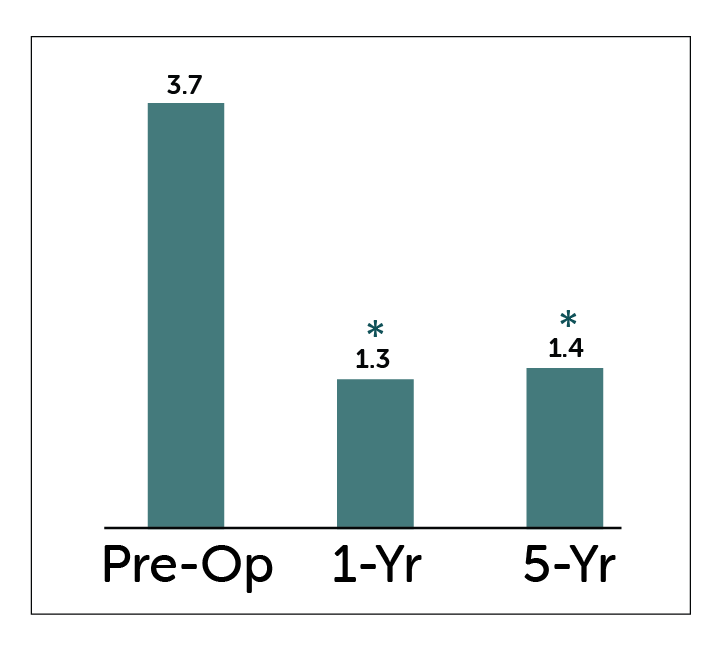
*P<0.05 compared to pre-operative values
This trial examined the safety and efficacy of Enterra Therapy in the treatment of chronic, intractable (drug-refractory) nausea and vomiting secondary to gastroparesis of diabetic etiology.
This trial examined the safety and efficacy of Enterra Therapy in the treatment of chronic, intractable (drug-refractory) nausea and vomiting secondary to gastroparesis of idiopathic etiology.
This independent study conducted by the French Ministry of Health examined efficacy of Enterra Therapy in the treatment of chronic, intractable nausea and vomiting, with or without gastroparesis.
This study examined the long-term (>10 years) safety and efficacy of Enterra Therapy in patients with intractable nausea and vomiting.
Zoll et al.
“Enterra Therapy does improve refractory nausea and vomiting in some patients with gastroparesis and may improve glycemic control, nutritional status, and quality of life, while reducing hospitalizations and medication use.”5
“Gastric Electric Stimulation (GES) may be considered for control of GP symptoms as a Humanitarian Use Device (HUD).”6
Enterra Therapy for treatment of chronic, resistant to medication nausea and vomiting associated with gastroparesis caused by diabetes or an unknown origin in patients aged 18 to 70 years: patients should always discuss potential risks and benefits of the device with their physician.
Authorized by Federal law for use in the treatment of chronic intractable (drug refractory) nausea and vomiting secondary to gastroparesis of diabetic or idiopathic etiology in patients aged 18 to 70 years. The effectiveness of this device for this use has not been demonstrated. What does this mean?