US control trial: idiopathic gastroparesis1
Objective
This trial examined the safety and efficacy of Enterra Therapy in the treatment of chronic, intractable (drug-refractory) nausea and vomiting secondary to gastroparesis of idiopathic etiology.
Design
Prospective, multi-center, double-blinded, 1:1 randomized controlled trial with cross-over study design across 6 centers in the United States.
Primary endpoint
Weekly vomiting frequency (WVF); post crossover ON phase relative to OFF phase.
Results
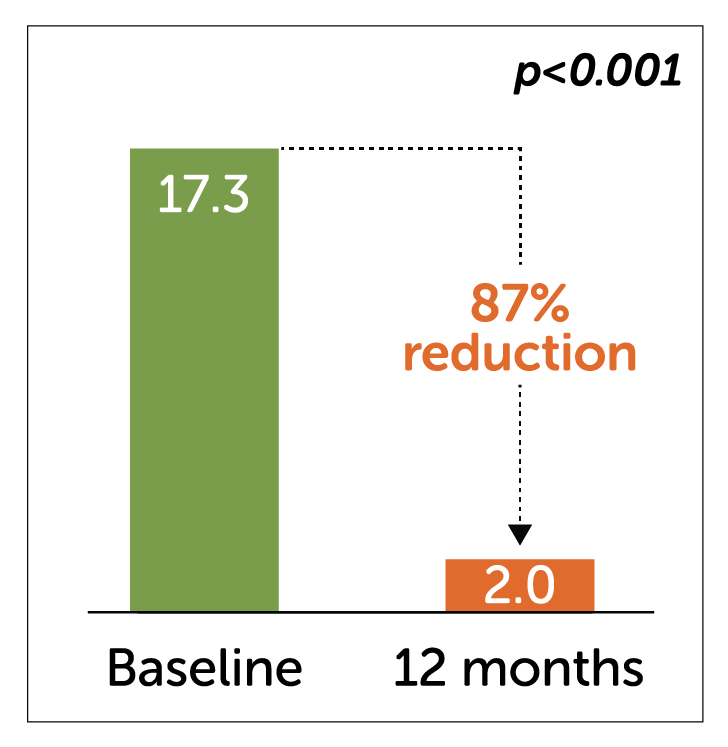
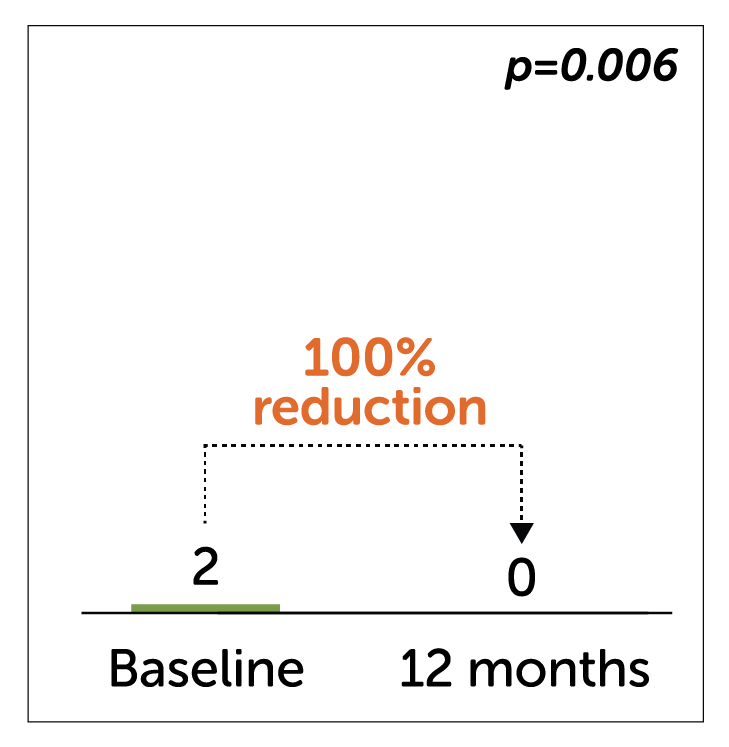
- In a study of 32 idiopathic gastroparetic patients, Enterra Therapy significantly reduced median WVF at 12 month follow-up.
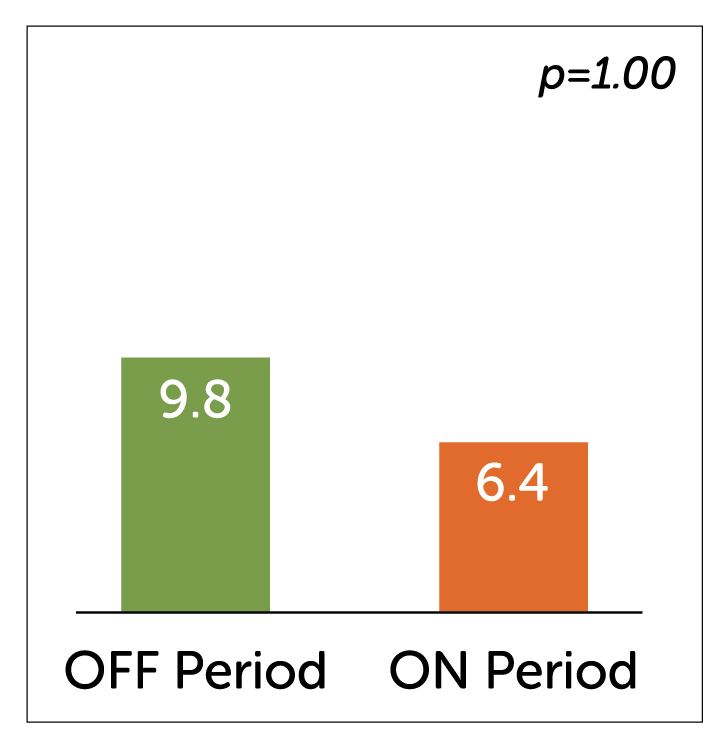
- Crossover periods showed a non-significant reduction in vomiting in the ON vs. OFF period which may have been due to the lack of washout effect of 6 weeks of stimulation that took place pre-randomization.
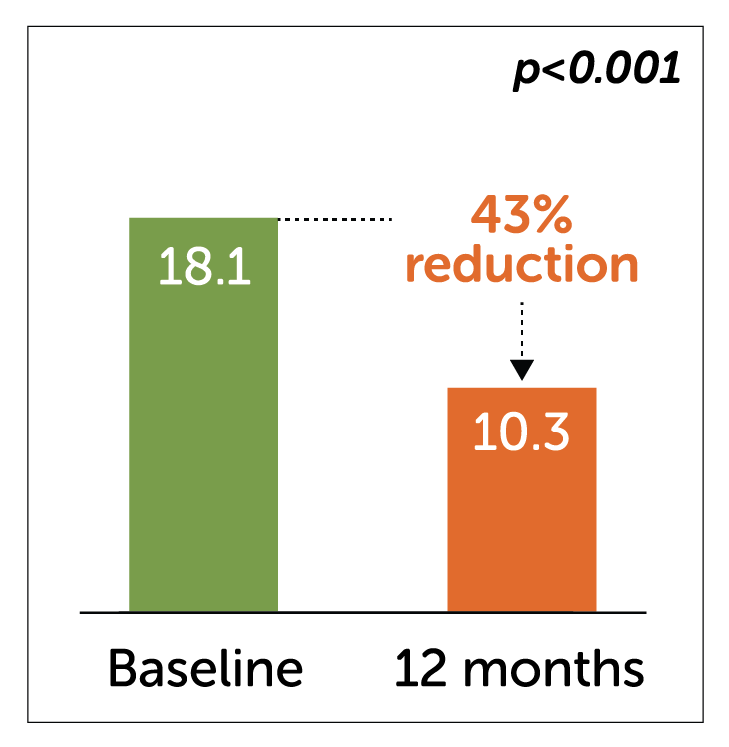
- Enterra Therapy significantly reduced key secondary endpoints of Total Symptom Scores and number of days hospitalized at 12 months.
Median Weekly Vomiting Frequency (WVF)
Total symptom score – severity
Hospitalization days
- McCallum RW, Sarosiek I, Parkman HP, Snape W, Brody F, Wo J, Nowak T. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013 Oct;25(10):815-e636.
